Fitting Guide for Extreme H2O 54
PROFESSIONAL FITTING GUIDE
FOR THE
EXTREME H2O® 54%
(hioxifilcon D)
Soft Contact Lens for Daily Wear
CAUTION: FEDERAL LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF
A LICENSED PRACTITIONER
Please read this guide carefully and follow the instruction so that you receive full satisfaction from your lenses.
The following symbols may appear on the label or carton:

Lot Number

Manufacturer

CAUTION: U.S. Federal law restricts this device to sale by or on the order of a physician

Expiration Date (Use by date)

Sterile by steam
Consult instructions for use

Authorized Reprsentative in the European Community
Indicates conformity with the essential health and safety requirements set out in European Directives
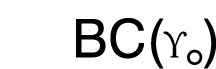
Base Curve

Diameter

Lens Power in Diopters
DESCRIPTION OF LENS
EXTREME H2O 54% (hioxifilcon D) soft contact lenses are hemispherical shells and are available as spherical lenses of the following
Extreme H2O 54% 13.6
PARAMETERS / VALUES
Diameter: 13.6
Center Thickness: 0.08 mm @ -3.00 Varies with power
Base Curve: Median 8.3
Power Range: -0.50 to -8.00 & +1.00 to +6.00 (0.50D steps after +4.00)
Extreme H2O 54% 14.2
PARAMETERS / VALUES
Diameter: 14.2
Center Thickness: 0.08 mm @ -3.00 Varies with power
Base Curve: Median 8.6
Power Range: -0.50 to -8.00 & +1.00 to +6.00 (0.50D steps after +4.00)
Extreme H2O 54% Toric
PARAMETERS / VALUES
Diameter: 14.2
Center Thickness: 0.145 mm @ -3.00 Varies with power
Base Curve: Median 8.6
Sphere Power: -0.25 to -6.00 & +0.25 to +4.00
Cylinder Power: 0.65 D (LC). 1.25 D (MC)
Axis:
LC: 15º, 75º, 90º, 105º, 165º, 180º
MC: 10º , 20º, 80º, 90º, 100º, 160º, 170º, 180º
The EXTREME H2O 54% (hioxifilcon D) soft contact lens is fabricated from hioxifilcon D, which is a non-ionic, copolymer of 2-hydroxyethyl methacrylate (2-HEMA) and 2,3-Dihydroxypropyl Methacrylate (Glycerol Methacrylate, GMA) and cross-linked with ethylene glycol dimethacrylate (EGDMA). It consists of 46% hioxifilcon D and 54% water by weight when immersed in normal saline solution buffered with either sodium bicarbonate or sodium perforate. The lens is available with a blue visibility handling tint, phthalocyanato (2) – (copper).
The Physical/Optical properties of the lenses are:
PARAMETER / VALUE
Refractive Index: 1.414 (hydrated)
Light Transmission – tinted: greater than 95%
Water Content: 54%
Specific Gravity: 1.300 (dry)
Oxygen Permeability (DK Value): 21 x 10 (-11) Fatt Units (cm²/sec)(ml O2/ml x mm Hg), ANSI Z80:2004 upraded polarographic method
ACTIONS
In its hydrated state, the EXTREME H2O 54% (hioxifilcon D) soft contact lens, when placed on the cornea, acts as a refracting medium to focus light rays on the retina. The toric lens provides a more even surface over the highly uneven astigmatic cornea and thus helps to focus light rays on the retina.
CAUTION
Due to the small number of patients enrolled in clinical investigation of lenses, all refractive powers, design configurations, or lens parameters available in the lens material were not evaluated in significant numbers. Consequently, when selecting an appropriate lens design and parameters, the eye care practitioner should consider all characteristics of the lens that can affect lens performance and ocular health, including oxygen permeability, wettability, central and peripheral thickness, and optic zone diameter.
The potential impact of these factors on the patient’s ocular health must be carefully weighed against the patient’s need for refractive correction. Therefore, the continuing ocular health of the patient and lens performance on the eye should be carefully monitored by the prescribing eye care practitioner.
INDICATIONS
The Extreme H2O 54% (hioxifilcon D) spherical soft contact lens for daily wear is indicated for the correction of visual acuity in aphakic or
not-aphakic persons with non-diseased eyes that are myopic or hyperopic. The lens may be worn by persons who exhibit astigmatism of 0.75
Diopters or less that does not interfere with visual acuity.
The Extreme H2O® 54% (hioxifilcon D) toric soft contact lens for daily wear is indicated for the correction of visual acuity in aphakic or notaphakic persons with non-diseased eyes that are myopic or hyperopic. The lens may be worn by persons who exhibit astigmatism of 10.00
Diopters or less.
Eye care practitioners may prescribe the lens for frequent/planned replacement wear, with cleaning disinfection and scheduled replacement.
When prescribed for frequent/planned replacement wear, the lens may be disinfected using a chemical disinfection system.
CONTRAINDICATIONS
DO NOT USE the EXTREME H2O DAILY (hioxifilcon D) soft contact lenses when any of the following conditions are present:
• Acute or subacute inflammation or infection of the anterior chamber of the eye.
• Any eye disease, injury, or abnormality that affects the cornea, conjunctive, or eyelids.
• Severe insufficiency of lacrimal secretion (dry eyes).
• Corneal hypoesthesia (reduced corneal sensitivity), if not-aphakic.
• Any systemic disease that may affect the eye or be exaggerated by wearing contact lenses.
• Allergic reactions of ocular surfaces or adnexa that may be induced or exaggerated by wearing contact lenses or use of contact lens solutions.
• Allergy to any ingredient, such as mercury or thimerosal, in solution which must be used to care for EXTREME H2O DAILY soft contact lenses.
• Any active corneal infection (bacterial, fungi, or viral).
• If eyes become red or irritated.
• Patients unable to follow lens care regimen or unable to obtain assistance to do so.
WARNINGS
Patients should be advised of the following warnings pertaining to contact lens wear:
PROBLEMS WITH CONTACT LENSES AND CONTACT LENS CARE PRODUCTS COULD RESULT IN SERIOUS INJURY TO THE EYE. It is essential that you follow your eye care practitioner’s directions and all labeling instructions for proper use of your lenses and lens care products, including the lens case. EYE PROBLEMS, INCLUDING CORNEAL ULCERS, CAN DEVELOP RAPIDLY AND LEAD TO LOSS OF VISION; THEREFORE, IF YOU EXPERIENCE ANY DISCOMFORT, EXCESSIVE TEARING, VISION CHANGES, REDNESS OF THE EYE, IMMEDIATELY REMOVE YOUR LENSES AND PROMPTLY CONTACT YOUR EYE CARE PRACTITIONER.
Daily Wear lenses are not indicated for overnight wear, and patients should be instructed not to wear lenses while sleeping. Clinical studies have shown that the risk of serious adverse reactions is increased when these lenses are worn overnight.
Studies have shown that contact lens wearers who are smokers have a higher incidence of adverse reactions than nonsmokers.
If a patient experiences eye discomfort, excessive tearing, vision changes, or redness of the eye, the patient should be instructed to immediately remove lens and promptly contact his or her eye care practitioner.
ALL CONTACT LENS WEARERS MUST RETURN FOR PERIODIC CHECK-UP VISITS AS RECOMMENDED BY THEIR EYE CARE PRACTITIONER.
FITTING PROCEDURE (SPHERICAL)
Patient Selection
The practitioner should first assess the patient’s needs and characteristics necessary to fit with Extreme H2O Monthly lenses. A through pre-fitting examination should be conducted to ensure the patient is a suitable candidate for soft contact lens wear.
Pre-fitting Examination
A pre-fitting examination is necessary to:
• Determine if the patient is a suitable candidate in terms of motivation, physical state, and willingness to comply with instructions concerning wear time and hygiene;
• Carefully evaluate the lids, lashes, conjunctival areas as well as the anterior segment of the eye for suitability for contact lens wear;
• Take ocular measurements for initial contact lens parameter selection; and
• Collect and record baseline clinical information to which post-fitting examination results can be compared.
A pre-fitting examination should include a case history, a spherocylindrical refraction, keratometric readings, tear assessment, and biomicroscopy of the anterior segment.
Initial Power Determination
The initial power selection should be as close as possible to the patient’s prescription after taking into account spherical equivalent and vertex calculations, if necessary. Remember to compensate for vertex distance if the refraction is greater than ± 4.00D.
Base Curve Selection
A well-fitted lens provides good movement, centration, and comfort. This can be achieved for the majority of patients with the 8.4 mm base curve. However, corneal curvature measurements should be performed to establish the patient’s baseline ocular status.
Place a lens on each of the patient’s eyes and allow a 15 minute period of adjustment and equilibration.
Characteristics of a Well-Fitted Lens:
A properly fitted lens will center and completely cover the cornea in all fields of gaze, allow sufficient lens movement to provide tear exchange under the lens during a blink in primary or upgaze, move freely when manipulated with the index finger on the lower lid nudging upward, and return to its properly centered position when released.
Characteristics of a Tight (Steep) Lens: A tight or steep fit may provide insufficient or no lens movement during a blink in primary or upgaze, resist movement if nudged upward with the index finger and/or cause fluctuating vision between blinks. If the contact lens is deemed to be steep fitting, do not dispense to the patient. A flatter lens (larger base curve) should be evaluated if available.
Characteristics of a Loose (Flat) Lens: A loose or flat fit may exhibit reduced comfort, decentration, excessive movement during the blink or in primary upgaze, and/or edge standoff. If the contact lens is deemed to be flat fitting, do not dispense to the patient. A steeper lens (smaller base curve) should be evaluated if available.
Final Lens Power Determination
After the lens fit is successful, a spherical over-refraction should be performed to determine the proper lens power to be dispensed.
Example:
Diagnostic lens: -3.00D
Over-refraction: -0.25D
Final lens power: -3.25D
FITTING PROCEDURE 54% TORIC LC (Low Cylinder)
FITTING PROCEDURE (TORICS)
See the fitting procedure above for spherical lenses. Most aspects of the fitting procedures are the same for all types of soft contact lenses, but there are additional steps to follow to assure the proper fit of toric lenses.
Orientation Guide Marks: The Extreme H2O 54% Toric lenses are marked at 6 o’clock and 20º off 6 o’clock on either side for ease in properly
orienting the lenses and observing rotation.
Determine Contact Lens Power:
The Extreme H20 54% Toric LC has a cylindrical power of 0.65 D and can be used for a cylindrical correction in the range of 0.5 up to 1.0 D. When the toric diagnostic lens does not have a power equivalent to the patient’s spectacle prescription, sphero-cylinder over-refraction will often be inaccurateand confusing. Therefore, it is usually preferable to use the spectacle prescription as the only basis for the contact lens power. The sphere of the spectacle prescription becomes the sphere power of the contact lens and the cylinder power is given by the LC lens as 0.67 D.
There are two exceptions:
• If spectacle cylinder power falls between available contact lens cylinder powers, prescribe the lesser contact lens cylinder power. The
sphere power can be increased -0.25D to compensate if desired. Of course this can vary depending on your interpretation of the patient’s
subjective responses. Example:
Spectacle Rx: -2.00 –1.00 x 180
Contact Lens Power Ordered -2.25 LC x 180
• When the spectacle lens power in any principle meridian is greater than 4.00D, the spectacle refraction should be adjusted to the corneal
plane. This can affect both the sphere and cylinder powers ordered. Example:
Spectacle Rx at 14mm vertex distance -6.00 -1.25 x 180
Contact Lens Power -5.50 -1.00 x 180
Contact lens power ordered -5.50 LC x 180
Determine Contact Lens Axis: Note the orientation of the guide marks relative to the vertical meridian. A popular method of axis compensating for axis misorientation is called “LARS” (Left Add, Right Subtract). Regardless of which eye the lens is on, if the rotation is left to the examiner, note the amount of rotation, add it to the refractive cylinder axis and order the resulting axis. If the rotation has stabilized to the right of the examiner, again note the rotation, subtract it from the refractive index and order the resulting axis. The guide marks can be used to help you calculate the axis of the desired Rx lens. Example:
Spectacle Rx: -2.50 –0.75 x 80
Rotation 20° to the left of the examiner
Final Lens Prescription -2.50 LC x 100
Round to nearest 30º axis increment (180, 30, 60, 90, 120, 150)
Final lens selection: -2.50 LC x 90
Evaluate Orientation of Final Rx Lenses: Once the final prescription has been determined, the orientation of the prescription should be the same as that observed for the lens used for fitting. For example, if the lens rotated clockwise 20° then the final prescription lens should also rotate clockwise 20°.
FOLLOW-UP EXAMINATIONS
• Within one week of lens dispensing
• After three weeks of lens wear
• After seven weeks of lens wear
• After each six-month period of lens wear.
At the follow-up examinations, the patient should report good subjective quality of vision. Adaptation to vision with Extreme H2O 54% (hioxifilcon D) soft contact lenses should occur almost immediately and should definitely be reported within the first (1 week) follow-up visit.
At these follow-up visits the practitioner should:
• Check distances and near acuity with lenses in place.
• Over-refract to verify lens prescription.
• Observe the position of the lens on the cornea. The lens should be centered and move on upward gaze and with a blink.
• Evert the lids to examine the tarsal conjunctiva and check for incidence of giant papillary conjunctivitis.
• Remove the lens. Check corneal curvature. There should be no substantial changes in either meridian.
• Perform a slit-lamp examination with and without Fluorescein. Check for corneal edema, corneal abrasion, vascularization, corneal infiltrates, and perilimbal injection. Reinsert the lens only after all residual Fluorescein has dissipated from the eye.
• Clean the lens with a surfactant cleaner, and examine for deposits, foreign bodies or physical imperfections of the lens surface.
LENS HANDLING
Wash and rinse hands thoroughly, making certain all soap residues have been rinsed away before drying with a lint-free towel. It is suggested to wet the lens while in the eye using lubricating and rewetting drops before removal of the lens. Care should be used not to pinch the lens when removing it from the eye. Pinching the lens can reduce the life of the lens.
Always start with the right lens first in order to avoid mixing the lenses. In removing the lenses, try to avoid touching the inside (concave) surface of the lens. It is possible, though not likely, that the lens might be inside out; therefore, check the lens by placing it on the index finger and examine its profile. If the edges of the lens tend to point outward, the lens is inside out. After removing the lens from its container assure that it is clean, clear and wet.
CLEANING & RINSING Based on practitioner recommendation, a surfactant cleaner can be used with Extreme H2O 54% hioxifilcon D soft contact lenses to ensure a clean lens surface. Follow the recommendations of the manufacturer of the cleaning solution. Thoroughly rinse both surfaces of the lens with a steady stream of rinsing solution.
CHEMICAL (NOT-HEAT) LENS CARE SYSTEM A sterile rinsing, storing, and disinfecting solution should be used to rinse and chemically disinfect Extreme H2O 54% (hioxifilcon D) soft contact lenses. After cleaning the lens, rinse with a liberal amount of fresh rinsing solution to remove loosened debris and traces of cleaner. The lens should then be placed in an appropriate lens storage case and filled with enough fresh disinfecting solution to completely submerge the lens. To ensure disinfecting, the lens must remain in the disinfecting solution for the period of time recommended in the disinfecting solution instructions for use. Before reinsertion, lenses should be rinsed with fresh sterile rinsing solution.
LENS CARE DIRECTIONS
Refer to Package Insert.
STORAGE
The Extreme H2O 54% (hioxifilcon D) soft contact lenses must be stored in the recommended solutions. If exposed to the air, the lenses will dehydrate. If a lens dehydrates, it should be soaked ONLY in a soft contact lens storage solution until it returns to a soft, supple state. It should not be put on an eye until it has been put through a complete disinfecting cycle and a thorough inspection.
LENS CARE PRODUCTS
The eye care practitioner should recommend a care system that is appropriate for hydrophilic soft contact lenses. Each lens care product contains specific directions for use and important safety information, which should be read and carefully followed.
RECOMMENDED WEARING SCHEDULE
Wearing schedule should be determined by the eye care practitioner. Close professional supervision is recommended to ensure safe and successful contact lens wear. If the patient complains of discomfort, decreased vision, ocular injection or corneal edema, the lens should be removed and the patient scheduled for examination. The problem may be relieved by putting the patient on a different wearing schedule or possibly by refitting the lenses. Patients tend to over-wear the lenses initially. It is important not to exceed the initial wearing schedule. Regular check-ups, as determined by the eye care practitioner, are also extremely important. The maximum suggested wearing schedule for Extreme H2O 54% (hioxifilcon D) soft contact lenses is as follows.
SUGGESTED WEARING SCHEDULE
Days / Continuous Hours
1 / 4
2 / 6
3 / 8
4 / 10
5 / 12
6 / 14
Until first follow-up examination @ 8 days / 14
All waking hours or according to the practitioner recommendation
Studies have not been completed to show that EXTREME H2O 54% (hioxifilcon D) soft contact lenses are safe to wear during sleep.
RECOMMENDED METHODS OF LENS INSERTION
1. The lens should be placed on the tip of the index finger of the dominant hand. Place the middle finger of the same hand close to the
lower lash and hold the lid down.
2. Use the forefinger or middle finger of the other hand to lift up the upper lid. Look straight ahead and gently place the lens directly on the
eye.
3. Gently release the lids and blink. The lens will center automatically.
4. If there is an initial foreign body sensation, the patient should look up to the ceiling and slide the lens off the cornea. Then the patient
should look down until the lens re-positions itself on the cornea. If the foreign body sensation persists, the patient should remove the lens,
rinse it with a recommended rinsing solution, and reinsert. If the foreign body sensation still persists, the patient should remove the lens.
5. Use the same technique or reverse hands when applying the other lens.
Alternate method of lens insertion
If patient is unable to insert the lens using this method, the eye care practitioner should provide an alternate method for lens insertion.
RECOMMENDED METHODS OF LENS REMOVAL:
Blink Method
The blink method is a safe way to remove the lens while avoiding folding or pinching the lens. Pinching increases the chances of lens splitting or tearing. This method is also useful to those patients who have difficulty touching their fingers to the lens while it is still on their eye.
Seat yourself at a table covered with a clean towel and lean over until you are looking directly down at the surface.
1. Wet eye using lubricating and rewetting drops.
2. Open eye wide and place opposite hand below the eye; palm up (open).
3. Place index finger on the outside edge of the upper lid and press eyelid upward above the contact lens.
4. Press the upper and lower lid margins against the eye, using the index and middle fingers of each hand.
5. At the same time, pull both lids out toward the ear.
6. Attempt to blink. The lens edge will be folded by the pressure of the eyelids. The lens will then pop out of the eye and either be stuck to
the lid(s), or you may catch the lens in the palm of your hand.
7. Remove lens from lid(s). Avoid pinching the lens.
Alternate method
1. Insert 1 – 2 drops of lubricating and rewetting solution in eye and wait 15 seconds.
2. Look up and hold down lower lid.
3. Slide lens onto white of eye (sclera) and gently lift off using thumb and forefinger at the widest point (3 & 9 o’clock) of the lens.
4. It is important not to crease or pinch the lens in the center or at the bottom edge to avoid damaging the material.
5. Repeat procedure for the other eye.
EMERGENCIES
The patient should be informed that if chemicals of any kind (household products, gardening solutions, laboratory chemicals, etc.) are splashed into the eyes, the patient should: FLUSH EYES IMMEDIATELY WITH TAP WATER FOR AT LEAST 15 TO 30 MINUTES AND IMMEDIATELY
CONTACT THE EYE CARE PRACTITIONER OR VISIT A HOSPITAL EMERGENCY ROOM WITHOUT DELAY.
REPORTING OF ADVERSE REACTIONS
All serious adverse experiences and adverse reactions observed in patients wearing Extreme H2O 54% (hioxifilcon D) soft contact lenses or experienced with the lens should be reported to:
Clerio Vision
7575 Commerce Court
Sarasota, FL 34243 USA
1-877-336-2482
HOW SUPPLIED
Each Extreme H2O 54% (hioxifilcon D) soft contact lens is supplied sterile in a sealed blister pack containing buffered normal saline solution. The blister pack is labeled with the base curve, power, diameter, manufacturing lot number, and the expiry date of the lens. Do not use if the blister is damaged or the seal is broken.
Manufactured and Marketed by
Clerio Vision
7575 Commerce Court
Sarasota, FL 34243 USA